Abstract
Introduction: A hypercoagulable state leading to increased risk for thrombotic events represents one of the most common complications observed in transfusion-dependent thalassemia (TDT) patients. TDT patients have increased frequencies of circulating activated platelets. However, there is no information so far if platelets from TDT patients can activate T cells.In the present study we examined the potential of T cell activation from platelets from TDT patients.
Methods: After written informed consent was obtained, peripheral blood mononuclear cells (PBMCs) were isolated from heparinized venous blood obtained from 50 patients with TDT (27 women and 23 men, mean age 38 years ) at the time of hospital admission just before their transfusion. We also analyzed 30 age-matched healthy subjects ( mean age 39,5 years) who served as the control group. Platelet rich plasma or plasma alone were isolated from all patients and healthy controls and used in mixed short term cultures with the isolated PBMCs. The membrane expression of CD69 was used as a marker for T-cell activation. We also analyzed the percentages of CD4+CD25+highCD127low+ cells representing the regulatory subset (Tregs) by flow cytometry. The soluble (s) P-selectin, soluble Urokinase-type Plasminogen Activator Receptor (suPAR) and the Growth Differentiation Factor-15 (GDF15) levels were also measured. The study was approved by the Ethics Committee of the "Aghia Sophia" University Hospital, Athens, Greece.
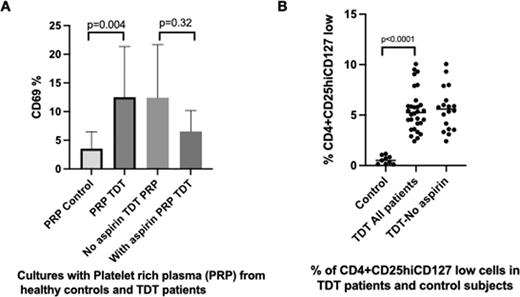
Results: We first examined T cell activation following incubation with platelets obtained from TDT patients. T cells treated with platelets from TDT patients showed statistically increased surface expression of CD69 compared to the T cells treated with platelets from healthy individuals (12.75±2.27 vs 4.03± 0.92 %, p=0.004, Figure 1A). When T cells were incubated with platelets from patients previously treated with aspirin, T cell activation was not as efficient as the activation observed in patients who were not treated with aspirin (6.53±2.11 vs 11.30±4.90 %, p=0.32, Figure 1A).Of note, patients with splenectomy showed increased T cell activation compared to patients with intact spleen (16.20±3.20 vs 8.15±1.96%, p=0.07). No T cell activation was observed following incubation with plasma alone, nor with platelets from healthy subjects. We next examined the percentages of CD4+CD25+high and CD127low cells (Tregs). TDT patients showed statistically significant increased levels of Tregs compared to healthy controls (5.47±0.38 vs 0.55±0.13 %, respectively p<0,0001, Figure 1B). Patients who were on aspirin on a daily basis showed slightly lower levels of Tregs, compared to the patients who were not treated with aspirin (5.25±0.6 vs 5.62±0.5% , p=0.64). Additionally, we observed a positive statistically significant correlation between the percentages of Tregs and the platelet-induced activated T cells in patients who were not treated with aspirin (p=0.009, r2=0.70). We next analysed the expression of sP-selectin, suPAR and GDF-15 in TDT patients. These molecules were selected as they are implicated in platelet activation. TDT patients showed increased levels of sP-selectin, suPAR and GDF-15 compared to healthy volunteers levels ( 174.4±12.67 vs 66.8±3.84 pg/mL respectively, p<0.0001, 3.39±0.15 vs 1.90±0.08 pg/mL respectively, p<0.0001, and 6633±215.9 vs 678.9±50.14 pg/mL respectively, p<0.0001). A positive, statistically significant correlation was observed between T cell activation after platelet incubation and sP-selectin levels (r2=0.3 p=0.03). A positive correlation was also detected between circulating activated T cell and suPAR levels (r2=0.56, p=0.01).
Conclusion: Our data concur with previous reports supporting the hypothesis that platelets play a role in the immune homeostasis. We show for the first time that platelets from TDT patients can activate T cells in vitro. This activation correlates with markers of platelet activation like sP-selectin and suPAR. TDT patients who showed increased platelet-induced T cell activation, showed also increased numbers of Tregs, perhaps in an effort to eliminate immune dysregulation, conceivably secondary to platelet activation.
Disclosures
Diamantopoulos:Novartis, Roche, Janssen, BMS: Honoraria. Kattamis:IONIS: Consultancy; BMS/Celgene: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Research Funding; VERTEX: Consultancy, Honoraria; ViFOR: Consultancy; AMGEN: Consultancy; Chiesi: Honoraria; AGIOS Pharmaceuticals: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal